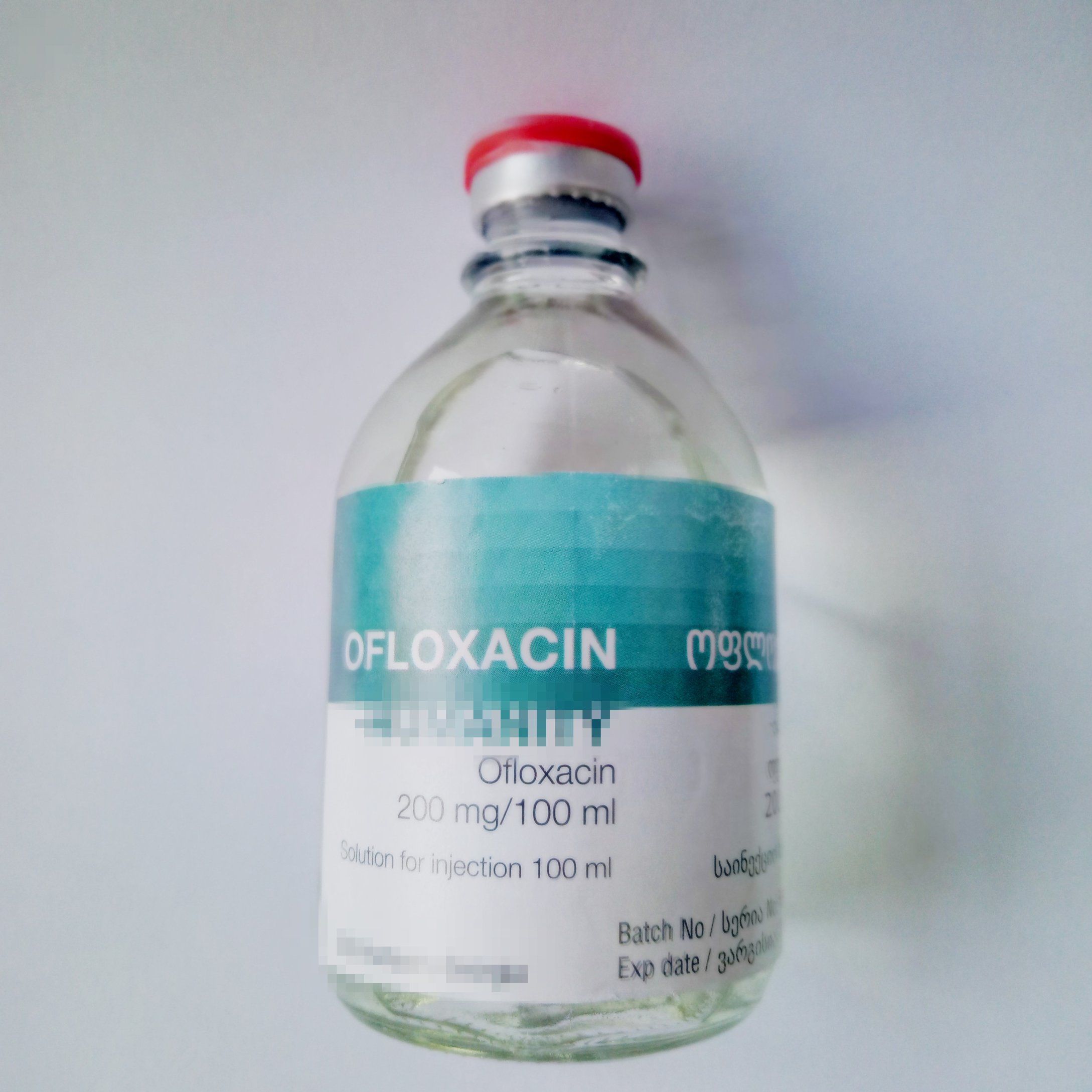
Product Specifications
| Prod Model: |
LMI02 |
| Type: |
Biological Products |
| Pharmaceutical Technology: |
Chemical Synthesis |
Product Description
Function: this product is applicable to sensitive bacteria:
1. Urinary tract infection, including simple, complex urinary tract infection, bacterial prostatitis, gonorrhoea urethritis, or cervicitis (including the production of enzyme strains).
2. Respiratory tract infection, including the acute infection of bronchial infection caused by sensitive gram-negative bacillus, and pulmonary infection.
3. Gastrointestinal infection caused by shigella, salmonella, escherichia coli, hydrophilic monocytosis and vibrio vibrio.
4. Typhoid.
5. Bone and joint infection.
6. Skin soft tissue infection.
Septicemia and other systemic infections.
chemical composition(+) - 9 - hydrogen fluoride - 2, 3-2-3 - methyl - 10 - (4 - methyl - 1 - piperazine) - 7 - oxygen generation - 7 h - pyridine and [1, 2, 3 - DE] - [1, 4] oxazine - benzene and 6 - carboxylic acid.
Usage:intravenous drip.
Adult dosage:| 1.Bronchial infection and pulmonary infection | 0.3g at a time, 2 times a day, for 7 to 14 days |
| 2.Acute single-thoroughness of urinary tract infection | 0.2g, 2 times a day, for 5-7 days |
| 3.Prostatitis | 0.3 g, 2 times a day, 6 weeks |
| 4.Simple gonorrhea | 0.4g at a time, single dose |
| 5.Typhoid fever | 0.3g at a time, 2 times a day, for 10 ~ 14 days |
The dose of pseudomonas aeruginosa infection or heavy infection can be increased to 0.4 g, 2 times a day.
Adverse reactions:1. Gastrointestinal reaction: abdominal discomfort or pain, diarrhea, nausea or vomiting.
2. The central nervous system can have dizziness, headache, lethargy or insomnia.
3. Allergic reaction: skin rash, itching of skin, occasional exudative polymorphous erythema and vascular neuroedema.
Photosensitive reactions are rare.
4. Occasionally:
(1) seizures, mental disorders, restlessness, confusion, hallucinations, tremors.
(2) hematuria, fever, rash and other interstitial nephritis.
(3) phlebitis.
(4) crystallization, especially in high dosage applications.
(5) joint pain.
5. A small number of patients can develop serum aminotransferase, higher blood urea nitrogen and lower blood cell leukocyte in the surrounding area, and the injection site can stimulate the symptoms, which are mild and transient.
Note:1. Every 0.2g of this product should not be less than 30 minutes.
2. Due to the fact that escherichia coli is more common to fluoroquinolone drug resistant, it should be used to take urine culture samples before administration, and then adjust the medication by referring to bacterial drug sensitivity.
3. Crystalline urine can occur at more than 7 days of high dose application or urine pH value.
In order to avoid the occurrence of crystal urine, it is advisable to drink more water and keep the urine volume at more than 1200ml in 24 hours.
4. Patients with renal function may need to adjust the dosage according to the kidney function.
5. Should avoid excessive exposure to sunlight when applying this product, if the photosensitive reaction needs to be stopped.
6. Hypohepatia, in the case of a severe (cirrhosis ascites) can reduce drug cleared, blood drug concentration increased, decreased liver and kidney are particularly, application after all needs to weigh the pros and cons, and adjust the dosage.
7. Patients with pre-existing central nervous system disorders, such as epileptic and epileptic patients, should avoid application.
Description:this product is yellowish green transparent liquid.
Contraindications:patients with allergies to this and quinolones are prohibited.
Pregnant women and lactating women: the animal experiment did not confirm that quinolones were deformed, but there was no clear conclusion on the study of pregnant women.
In the light of the fact that this drug may cause joint lesions of juvenile animals, pregnant women should not be allowed to breastfeed.
Children's medicine:the safety of this product in infants and children under the age of 18 has not been determined.
But this product is used in several young animals, can cause joint pathological changes.
Therefore, it should not be used for children and adolescents under 18 years of age.
Old age drug: elderly patients often have kidney function decline, because this product part is excreted by the kidney, need to reduce the application.
Drug interactions:1. Urea can reduce the solubility of this product in urine, resulting in crystal urine and renal toxicity.
2. Share the quinolone antibacterial and theophylline class could be explained by combined with cytochrome P450 competitive inhibition, seriously reduce the liver to eliminate theophylline class, blood elimination half-life ,blood drug concentration increases, theophylline poisoning symptoms, such as nausea, vomiting, tremor, anxiety, excitement, convulsions, heart palpitations and so on.
Although the metabolism of the product has little effect on the metabolism of the tea alkali, the concentration and the adjustment dose of theophylline should be determined.
3. When the product is combined with cyclosporine, the blood concentration of cyclosporin can be increased and the concentration of cyclosporine blood must be monitored and the dosage is adjusted.
4. Although the anticoagulant effect of this product and the anticoagulant warfarin is enhanced, it should be closely monitored for the prothrombin time.
5. It can reduce the secretion of the renal tubules by about 50%, and can be toxic because of the increase of blood concentration.
6. This product can interfere with the metabolism of caffeine, which results in the elimination of caffeine, the extension of the blood removal of the half-life (t1/2 beta), and the possible toxicity of the central nervous system.
Pharmacological effects:this product has antibacterial function, especially for aerobic gram-negative bacilli high antibacterial activity, good antibacterial effect in vitro on the following bacteria: most of enterobacteriaceae bacteria, including citron acid bacillus, sewer, gas, e. coli, e. coli, e. coli, klebsiella bacteria genera, proteus, salmonella, shigella, vibrio genera, yell, bacteria, etc.
There are also antibacterial activity of multidrug-resistant bacteria.
Antibacterial activity of neisseria gonorrhoeae, haemophilus and mora is highly antibacterial.
Most strains of pseudomonas aeruginosa, such as pseudomonas aeruginosa, were antibacterial.
This product has antibacterial activity of staphylococcus methoxicillin, and is only moderately antibacterial to streptococcus pneumoniae, hemolytic streptococcus and enterococcus faecalis.
The antibacterial action of chlamydia trachomatis, mycoplasma and legionella bacteria was found to have antimicrobial activity against mycobacterium tuberculosis and atypical mycobacteria.
The antibacterial activity of anaerobic bacteria is poor.
It is a bactericide that inhibits DNA synthesis and replication by acting on A subunit of bacterial DNA helicase, which causes bacterial death.
Our company supplies different kinds of products. High quality and reasonable price. We stick to the principle of "quality first, service first, continuous improvement and innovation to meet the customers" for the management and "zero defect, zero complaints" as the quality objective. To perfect our service, we provide the products with good quality at the reasonable price.
Wuhan Chuanfan Import and Export Trade Co., Ltd. (Hereinafter called "Chuanfan") focus on independent product research and sales, whose holding company is Wuhan Fuxing Bio-Pharmaceutical Co., Ltd. Since its establishment, Chuanfan actively expands to overseas markets, including the Middle East, Europe and South America, Africa is one of our key development areas. In this year we established Kaiyuantang (U) Limited in Kampala, Uganda, and have obtained all certificates, including market authorization license on Drugs, and have submitted drug registration application to NDA, including narcotics, psychotropic drugs and other special medicine and we expect to pass acceptance for GMP inspection in May this year.